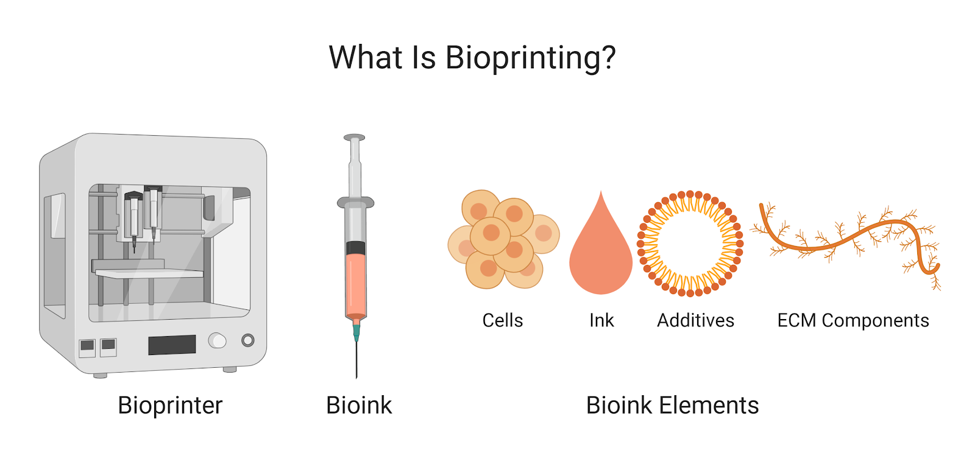
– by Minh-Tam Tran Le, Athena Olszewski and Keva Li
1. Introduction to Bioprinting
Definition
Bioprinting: the use of biomaterials and 3D printing technology to create structures and patterns for research or product development
Principles for Use
Different types of printers and ink allow for many different applications and high degree of control for user
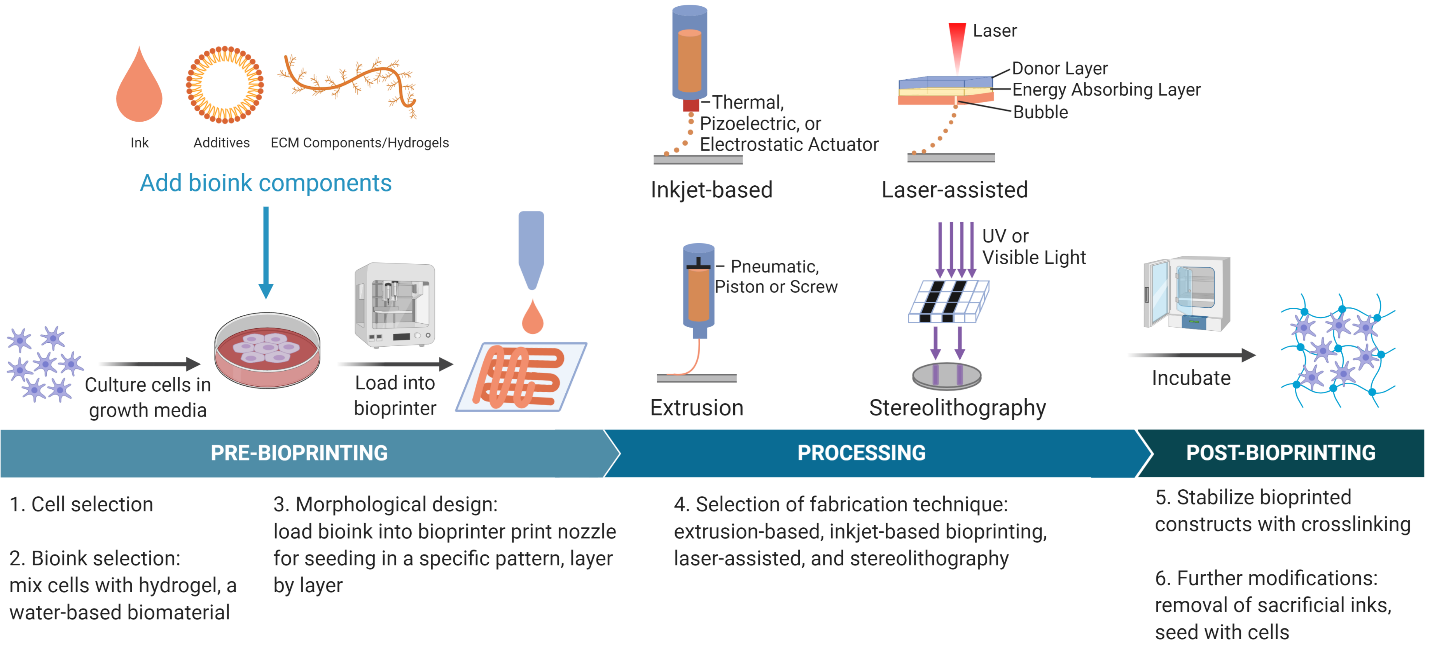
Many methods of bioprinting: extrusion-based, inkjet-based, stereolithography, laser-assisted bioprinting, fused-deposition modeling, selective laser sintering
Similar methods include: Biopatterning, cell bioprinting, scaffold printing, biomolding, photolithography-based bioprinting, and bioassembly
Applications
Tissue & Organ Engineering
Traditional method: cell culture over scaffolds or culturing organoids from tissue samples; difficult to achieve microstructure of tissues, slow
Bioprinting advantages: micron scale level of printers offers more control and speed in recapitulating native tissue structures
Screening/High-throughput Assays
Traditional method: multi-well plates seeded and tested by hand; slow and difficult to test a high number of samples and conditions
Bioprinting advantages: automation can manage more samples and conditions with higher control and speed
Biomedical studies and biomimicry
Traditional method: uses animal or cell models; difficult to hone in on individual biomolecules and create highly biomimetic models in vitro
Bioprinting advantages: high flexibility in bioink type allows for high control over experimental design and microenvironment structure
Synthetic biology
Traditional method: engineered organisms like bacteria limited in applications to materials that can support them
Bioprinting advantages: multiples types of bioink can create films or particles to capture organisms for their desired applications
Table 1. Pros and cons of bioprinting.
| Pros | Cons |
| Increased reproducibility | High cost |
| High resolution | |
| Flexible design capabilities | |
| Faster fabrication | |
| Decreased reliance on animal models | |
| Higher control over mechanical microenvironment | |
| Longitudinal studies enabled by printed perfusable vasculature |
2. Commercially Available Bioprinters (2020)
Table 2: Comparing Bioprinters
| Tissue Scribe | Inkcredible | BIO X6 | Lumen X | FELIX BIOprinter | Allevi 1 | Allevi 2 | Allevi 3 | Brinter-3D BioPrinter | BIO X | Biopixlar | BioAssemblyBot (BAB) | Regenova | NGB-R | |
| Manufacturer | 3DCultures | CellInk | CellInk | CellInk | FELIX printer | Allevi | Allevi | Allevi | Brinter | CellInk | Fluicell | Advanced Solutions | Cyfuse | Poietis |
| Price ($ unless noted) | 1,499 | 4,999 | 11,239 (EU) | 24,900 | 39,000 | 99,995 and up | 159,990 | 250,000-350,000 | ||||||
| Head mounts | 1 | 2 | 6 | 1 | 2 | 1 | 2 | 3 | 1 | 3 | 3 | 1 | 1 | 1, 2, or 3 |
| Compatible with various heads | No | Yes | Yes | No | No | No | No | No | Yes | Yes | No | Yes | No | Yes |
| Print pressure range | Up to 700kPa (set range of 5-400kPa) | N/A | 20.7-689.4kPa | 6.9-827.4kPa | 6.9-827.4kPa | up to 600kPa | up to 700kPa | N/A | N/A | up to 300kPa | ||||
| Print Precision | 100um (xy) z 0.04375 | 100um (layer) 10um(xy) 2.5um(z) | 50um (xy) 5um (z) | 50um(layer), 1.6um (xy), 0.15um (z) | 7.5um(xy) 1um(z) | 5um(xy) 1um(z) | 1um(xy) 1um(z) | 15um (xy), 20um (z) | 1um | 150um | N/A | 100um (filament) 100nl(droplet) | ||
| Printbed temp | up to 60C | 4-60C | RT-37C | temp control | RT | RT | RT-60C | 5-60C | RT | |||||
| Printhead temp | up to 70C | Depends on head | Depends on head | N/A | 2-50C | 4-160C | RT-160C | 4-160C | Avaliable | 4-250C | Depends on head | RT | 4-60C (optional) | |
| Germicidal light | No | No | Yes | No | No | No | No | No | Yes | Yes | Optional | Yes | ||
| Interface | Screen (not touch) | Touch screen | Touch screen or direct to computer | Touch screen | Touchscreen | No screen | No screen | No screen | No screen | Touch screen | Gamepad control | Touchscreen | Touchscreen | |
| UV crosslinking | No | 365 nm (405 nm optional) | 365 and 405 nm | 405nm | Avaliable | 365 and 405 nm | 405 nm(std) 365 nm (op) | 365 and 405 nm | Avaliable | 365 and 405 nm | Yes | No | No | 365 and 405 nm |
| File types | Gcode | STL/OBJ/AMF | .STL, Gcode | .STL | .STL, Obj, 3MF | .STL | .STL | .STL | .STL | .STL, Gcode | .bio | |||
| Connectivity | microSD card, micro USB | USB, SD-card | USB, micro SD, ethernet, wifi | Ethernet | Ethernet, wifi, USB | USB, ethernet | USB, wifi | |||||||
| Size | 25.6×28.7×19.1cm | 33x37x38 cm | 47.65×78.1×35.45cm | 2443x41cm | 43x39x55cm | 30×28.3×27.5 cm | 31.3×31.1×30.8cm | 46.7×38.8x36cm | 55x75x65cm | 48x44x35.5cm | 80x70x57cm | 93.5x75x134.3cm | 134×82.5x174cm | 135x89x275cm |
| Weight | 7.26kg | 20kg (shipping weight) | 30kg | 9kg | 11.5kg | 7kg | 8.6kg | 21.8kg | 35kg | 17kg | 165 kg | 450kg | 400-450kg | |
| Build volume | 10x12x8cm | 13x8x5cm | 6.4x4x4cm | 9x6x13cm | 9x6x13cm | 9x6x13cm | 30x19x10cm | 13x9x7cm | 30x19x10cm | 25x30x25cm | Limited by needle array size | |||
| Max speed | 20mm/s | reccommended 20mm/s | 100mm/s | 400 mm/s | N/A | 1-20mm/s | ||||||||
| Type | Extrusion | Extrusion | Extrusion | Projection Stereolithography | Extrusion | Fused Deposition Manufacturing | Fused Deposition Manufacturing | Fused Deposition Manufacturing | Extrusion | Extrusion | Mirofluidic hydrodynamic confined flow | Additive and contour, extrusion | Cell mounter on needle array | Laser Assisted micro-valve and bio-extrusion |
| Notes | Includes Simplify3D liscence, elecro-mechanic pressure | Camera, automatic printer head changing | 6 axis robotic arm, live feed camera | Spheroid diameter 400-600um | Includes 6 axis robotic arm, built in microscope | |||||||||
| Research examples | [1] | [2] | [3] | [4] | [5] | [6] | [7] |
[1] A. Motealleh, B. Çelebi-Saltik, N. Ermis, S. Nowak, A. Khademhosseini, N.S. Kehr, 3D printing of step-gradient nanocomposite hydrogels for controlled cell migration, Biofabrication. 11 (2019). https://doi.org/10.1088/1758-5090/ab3582.
[2] D. van der Valk, C. van der Ven, M. Blaser, J. Grolman, P.-J. Wu, O. Fenton, L. Lee, M. Tibbitt, J. Andresen, J. Wen, A. Ha, F. Buffolo, A. van Mil, C. Bouten, S. Body, D. Mooney, J. Sluijter, M. Aikawa, J. Hjortnaes, R. Langer, E. Aikawa, Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics, Nanomaterials. 8 (2018) 296. https://doi.org/10.3390/nano8050296.
[3] P. Koti, N. Muselimyan, E. Mirdamadi, H. Asfour, N.A. Sarvazyan, Use of GelMA for 3D printing of cardiac myocytes and fibroblasts, J. 3D Print. Med. 3 (2019) 11–22. https://doi.org/10.2217/3dp-2018-0017.
[4] G. Ying, N. Jiang, S. Maharjan, Y. Yin, R. Chai, X. Cao, J. Yang, A.K. Miri, S. Hassan, Y.S. Zhang, Aqueous Two‐Phase Emulsion Bioink‐Enabled 3D Bioprinting of Porous Hydrogels, Adv. Mater. 30 (2018) 1805460. https://doi.org/10.1002/adma.201805460.
[5] G. Montalbano, G. Molino, S. Fiorilli, C. Vitale-Brovarone, Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds, J. Eur. Ceram. Soc. (2020). https://doi.org/10.1016/j.jeurceramsoc.2020.02.018.
[6] H. Kizawa, E. Nagao, M. Shimamura, G. Zhang, H. Torii, Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery, Biochem. Biophys. Reports. 10 (2017) 186–191. https://doi.org/10.1016/j.bbrep.2017.04.004.
[7] V. Keriquel, H. Oliveira, M. Rémy, S. Ziane, S. Delmond, B. Rousseau, S. Rey, S. Catros, J. Amédée, F. Guillemot, J.C. Fricain, In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications, Sci. Rep. 7 (2017) 1–10. https://doi.org/10.1038/s41598-017-01914-x.
3. Common Bioinks
Table 3.1 Summary of Common Components
| Bioink Component | Description | Tissue Engineering Applications | Pros | Cons | Price (for smallest size offered) | Related bioinks | Notes | References |
| Agarose | Polysaccharide extracted from seaweed | Cartilage | Non-toxic crosslinking, high stability | Not degradable, poor cell adhesion | $93 per 5g | Agarose/collagen, agarose/collagen/alginate, agarose/fibrinogen, carboxylated agarose | Displays thermal hysteresis | [1] |
| Alginate | Biopolymer derived from brown algae | Vascular | Mild crosslinking conditions (Ca2+), rapid gelation, high biocompatibility | Slow degradation kinetics, poor cell adhesion, poor post-printing stability | $56 per 100g | Alginate/cellulose, alginate/RGD, alginate/cellulose/calcium phosphate, alginate/cellulose/RGD | Cell adhesion can be improved with addition of cellulose or RGD, can be Usesd as a sacrificial material | [2] |
| Chitosan | Polysaccharide from shellfish | Cartilage, skin, and bone | High biocompatibility, antibacterial properties | Slow gelation rate | $359 per 2g (high purity, 100 kDa) | N/A | For applications where shellfish allergies may limit Uses of chitosan, fungus derived chitosan is available | [3] |
| Collagen | Structural protein from skin and connective tissue | Skin, bone, and liver | High biological relevance | Acid-soluble | $53 per 1g (Type I, V) | Type I-type XII collagen | N/A | [4] |
| Decellularized ECM | Isolated ECM from native tissue | Adipose, heart, cartilage, and liver | High biological relevance, tissue- specific, high cell survival | Undefined and inconsistent, loss of native ECM organization, low stability |
$87 per 1mL | Matrigel/Geltrex/Cultrex (sarcoma basement membrane ECM) | ECM composition is highly variable batch to batch (may want to order large amounts of one batch) | [5] |
| Fibrin/Fibrinogen | Protein formed during blood clotting | Vascular, cartilage | High biological relevance, rapid gelation | Limited printability | $210/205 per 1g | Fibrinopeptide A&B | Fibrin can be made from fibrinogen | [6] |
| Gelatin | Protein derived from partial hydrolysis of collagen | Vascular and cartilage | High biocompatibility, high water solutbility, thermally reversible gelation, high morphological stability | Poor shape fidelity, limited rigidity, issue of cross-linking agent removal and cytoxicity | $28 per 100g | Gel-MA, GelAlg-Vis | Gel-MA is most common bioink form (semi-synthetic hydrogel: biocompatible, biodegradable, photopolymerizable) but is more expensive (~$200 per gram) | |
| Gellan Gum | Polysaccharide from bacterium Sphingomonas elodea | Bone, cartilage, brain, lung | Shear thinning behavior, high gelling efficiency at physiological temperature | Brittle, poor stability | $54 per 100g | Phytagel, Gelzan | Mixed with RGD for brain applications, with Gel-MA for cartilage | |
| Hyaluronic Acid | Non-sulfated GAG found in connective, epithelial, and neural tissue | Osteo-chondral, vascular, cartilage | Fast gelation, promotes cell proliferation, good cell viability | Poor post-printing stability | $79 per 1g | Methacrylated HA (PhotoHA; Allevi) | Usually Usesd with other components (other ECM proteins, alginate, Gel-MA, etc.) to provide binding sites and stability | |
| Hydroxyapatite | Mineral found in teeth and bones | Bone | High strength and rigidity | Low printability, limited tissue specificity | $113 per 25g | Hydroaxyapatite particles (HAp) | Particle form mixed with Gel-Ma and HA-Ma for bone bioink | |
| PCL/PLA/PLGA | Biodegradable thermoplastic polymers/copolymers | Bone | High strength and rigidity | Low cell adhesion and proliferation | $48 per 5g (average Mn 80,000) | N/A | Slow degradation Usesful for drug delivery applications | |
| PEG | Synthetic polyether | Vascular and bone | Inert, high mechanical properties | Low viscosity, poor cell viability | $36.90 per 5g (average Mn 400) | PEG-diacrylate and methacrylate | Usesd as blend with other components (alginate, collagen, etc.) to increase mechanical properties | |
| Pluronic F127 | Poly(ehtylene oxide) and poly(propylene oxide) block copolymer | Nerve and vasculature | Printable at room temperatures, shear thinning material | Not suitable for long-term cell culture | $53 per 250g | N/A | Thermosenstivitiy depends on concentration of bioink, can be Usesd as a sacrificial material, | |
| Silk/Fibroin | Protein fiber obtained from silkworms or spiders | Cartilage, skin | High printability, high structural fidelity | Poor cell viability | $290 per 20mL | N/A | Mix with gelatin for cartilage engineering, Usesd mainly in scaffold applications | |
| Notes: pricing per gram is for powders, pricing per mL is for pre-made bioinks | ||||||||
Table 3.2 : Bioink Additives
| Bioink Additive | Description | Example Applications | Reference |
| Cryo bioink | Has two types: 1) Can utilize liquid to solid phase change of a composite hydrogel ink by rapidly cooling the ink below its freezing point which is then Usesd to create 3D strctures with complexity but soft tissue mechanical properties. 2) Printing nanodroplets for cryopreservation | 1) Complex 3D printing of soft tissue structures i.e. brain and lung. 2) Preserve cells, seek to reduce cryo-based harm associated with bulk volumes in traditional cryopreservation | [7], [8] |
| Genetically engieneered phage | Phages can be modified to include molecules (i.e. cell binding peptides) to achieve desired bioink properties | M13 phages have been modified to include integrin binding and calcium binding domains on their surfaces, then mixed with alginate, to improve alginate’s cell viability and adhesion | [9] |
| Conductive inclusions | Conductive inclusions can be in the form of layers, flakes, (gold/silver) nanoparticles, etc: will make electrically conductive bioinks fappropriate or stimuli-based testing | Graphene is a common conductive inclusion; has promoted cell viability and proliferation in many stem cell types and conductivity allows stimulation of cardiac cells | [10] |
| Magnetic inclusions | Magnetic nanoparticles are the common form of additive, allowing control over microstructure of the printed structure through manipulating a magnetic field, allows for dynamic control of bioink as magnetic-based properties are reversible through removal of magnetic field | Magnetic iron oxide in alginate controls viscosity, depending on concentration; are Usesful for highly controlled positioning of molecules within printed structures; Gold nanorodes have been shown to improve cardiac function | [9] |
| Biomolecules | Includes growth factors, proteins, hormones, etc: will promote cell proliferation, survival, tissue formation; can be specific to type of tissue | VEGF has been included for vascularization tissue engineering, either directly added into the printed scaffold or in a nanoparticle for controlled release | [9] |
| Peptides | Ultrashort peptides (unclude trimer, tetramer, and hexamers) have stimuli-responsive gelation and self-assembly properties that allow rigid bioink with good shape fidelity. | Tissue engineering applications (promotes alignment and elongation within a week for MSCs ecanpsulated in the peptide based bioink), drug delivery, and therapeutic screenings | [9] |
Table 3.3: Crosslinking Methods
| Bioink Component | Crosslinking Methods | Common Materials Needed | Pros | Cons | Notes | Reference |
| Agarose | Physical crosslinking | Cannot undergo physical crosslinking inherently; mix with chitosan and crosslink with dextrins | Cheap and easy to accomplish | Poor long term stability and mechanical properties | Agarose alone gels as it cools. In chitosan mixtures, oxidized dextrins have been indicated to have low cytotoxicity | [11] |
| Alginate | Physical crosslinking | Divalent cation source, usually bath/solution of calcium chloride (rapid and poorly controlled gelation but cheap), calcium sulfate, calcium chloride (lower solubilites result in slower gelation) | Cheap and easy to accomplish | Limited long-term stability in physiological conditions: divalent ions released over time, may promote hemostasis | Low temperatures or glucono-δ-lactone is Usesd to dissociate calcium cations to slow gelation. Slower gelation produces more uniform structures and greater mechanical integrity. Structure heavily depends on chemical structure of alginate. | [12] |
| Chemical crosslinking | Poly(ethylene glycol)-diamines of various molecular weights, or methacrylate for photo cross-linking via exposure to a laser (argon ion laser, 514 nm) for 30 seconds in the presence of eosin and triethanol amine | Improve physical properties | Reagents (including light sensitizer) may be toxic, can result in significant elastic deformation due to inability for bonds to dissociate | Unreacted toxic crosslinking reagents may need to be removed | ||
| Thermal crosslinking | Cannot undergo photocrosslinking inherently, N-isopropylacrylamide (NIPAAm) and poly(ethylene glycol)-co-poly(ε-caprolactone) (PEG-co-PCL) macromer copolymerized with sodium alginate via UV irradiation | Adjustable swelling properties | Alginate is not inherently thermosensitive, so copolymerization process requires extra time | Copolymerization results in semi-interpenetrating polymer network.Thermal sensitivity good especially in drug delivery applications due to quick modulation of gel for drug release | ||
| Cell crosslinking | Cells added to a RGD-modified alginate solution to form a uniform disperson and generate cross-linked network | Can create long and reversible network formation without chemical cross-linking agents, can provide additional mechanical inegrity | Alginate is not inherently good for cell adhesion, needs to be modified with cell adhesion ligands (RGD) which can be expensive | Non modified alginate solutions will result in cell aggregation and a non-uniform structure. Governed by weak ligand-receptor intreactions, so shear force can break structure | ||
| Chitosan | Physical crosslinking | Common physical crosslinkers: citric acid, dextran sulfate, or phosphoric acids | Cheap and easy to accomplish, creates flexible IPN | Limited long-term stability in physiological conditions: divalent ions released over time, may promote hemostasis | Cross-linker interlaces with chitosan to form interpenetrating polymer network (IPN) formation: tetraethyl orthosilicate identified to make IPN flexible and mechanically strong, good for chitosan membranes for wound dressings | [13] |
| Chemical crosslinking | Common chemical crosslinkers: glutaraldehyde, formaldehyde, tripolyphosphate, polyaspartic acid sodium salt | Improve phsyical properties | Reagents may be toxic | Unreacted toxic crosslinking reagents may need to be removed | [14] | |
| Collagen | Photocrosslinking | Cannot undergo photocrosslinking inherently, needs to be modified to be methacrylated, then crosslinked with UV light | Ease of treatment | Component needs to be methacrylated first, UV/gamma ray may damage cells | N/A | [15] |
| Chemical crosslinking | Common chemical crosslinkers: glutaraldehyde (GA), carbodiimide, diisocyanate, and acylazide | Improve phsyical properties | Reagents may be toxic | Glutaraldehyde is popular for biomaterial applications due to ease of Uses but can be toxic. Carbodiimides showed good initial but poor long term ability to promote cell infiltration | [16] | |
| Decellularized ECM | Varied | Crosslinking agents depend on the composition of the bioink, as it is a mixture of many types of other bioink components. | Depends on crosslinking type, see other components | Depends on crosslinking type, see other components | Matrigel, a popular ECM bioink, contains collagen, so could be crosslinked to an extent using collagen crosslinking agents; inks must be cooleged as gelation occurs at room temperature | [15] |
| Fibrin/Fibrinogen | Chemical crosslinking | Factor XIII/protransglutaminase incubated with fibronogen with calcium ions (>=50 uM), rate enhanced in presence of thiols | Improve phsyical properties | Reagents may be toxic | N/A | [17] |
| Gelatin | Physical crosslinking | Physical methods: Dehydrothermal and ultraviolet radiation treatments | Cheap and easy to accomplish | Poor long term stability and mechanical properties | Works by transitioning gelatin chains from coil to triple helices, crosslinking is thermo-reversible | [18] |
| Chemical crosslinking | Enzymes: transglutaminase, tyrosinases, and horseradish peroxidases. Chemical agents: glutaraldehyde, carbodiimides, genipin, bis(vinyl sulfonyl)methane | Improve physical properties | Reagents may be toxic | Unreacted toxic crosslinking reagents may need to be removed | [19] | |
| Gellan Gum* | Physical crosslinking | Temperature variation, divalent cations | Cheap and easy to accomplish | Limited long-term stability in physiological conditions: divalent ions released over time | N/A | [20] |
| Photocrosslinking | Cannot undergo photocrosslinking inherently, needs to be modified to be methacrylated, then crosslinked with UV light | Ease of treatment | Reagents may be toxic | Unreacted toxic crosslinking reagents may need to be removed | [21] | |
| Hyaluronic Acid* | Thermal crosslinking | Cannot undergo thermal crosslinking inherently, graft PNIPAAM onto HA and crosslink with heat | Increase control, improve physical properties | Hyaluronic is not inherently thermosensitive, so grafting process requires extra time | Good for drug delivery applications, microspheres often embedded into matrix and released through thermal control. Can mix with Pluronic to create a thermo-sensitive copolymer | [22] |
| Chemical crosslinking | Uses glutaraldehyde (GTA) [most common]; or 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide (EDC), poly(ethyelene glycol) diglycidyl ether (EX 810), divinyl sulfone (DVS), butanediol diglycidyl ether (BDDE) to modify hydroxyl or carboxyl groups | Improve physical properties | Reagents may be toxic | N/A | [23] | |
| Autocrosslinking | Uses mix of inter- and intra-molecular esters of hyaluronic acid, carboxyl groups are esterified with hydroxyl groups of another hyaluronic acid molecule | No need for additional reagents | Increased concern over reaction conditions | Level of crosslinking controlled through changes in reaction conditions | [24] | |
| Photocrosslinking | Initial crosslink with a glycidylether, Uses photosensitizer methylene blue. | Ease of treatment | Removal of photosensitizer, UV/gamma ray may damage cells | N/A | [25] | |
| Hydroxyapatite* | Chemical crosslinking | Cannot undergo chemical crosslinking inherently, mix with gelatin to crosslink using glutaraldehyde | Improve physical properties | Reagents may be toxic | Usually incorporated into inks in addition to ECM or as nanospheres for bone-related applications | [26] |
| PCL/PLA/PLGA | Photocrosslinking | UV light in the presence of a photoinitiator (i.e. BAPO) | Ease of treatment | Removal of photoinitiator, UV/gamma ray may damage cells | Initiator concentration can module physical properties of networks | [27] |
| PEG* | Photocrosslinking | Cannot undrgo photocrosslinking inherently, possible when modified to be PEG-diacrylate and methacrylate, crosslinked with UV light | Ease of treatment | Component needs to be methacrylated first, UV/gamma ray may damage cells | PEG can be modified and act as the cross-linker (for PEG, gelatin, gelatin/fibrinogen, gelatin/collagen bioinks) via the conjugated amine groups to improve mechanical properties | [28] |
| Pluronic F127* | Physical crosslinking | Cannot undergo physical crosslinking inherently, mix with gelatin to crosslink using mTG | Cheap and easy to accomplish | Poor long term stability and mechanical properties | Can undergo reversible thermal gelation, not crosslinking: is inherently thermosensitive | [29] |
| Silk | Chemical crosslinking | Uses a homobifunctional protein crosslinker: i.e. O’O‐bis[2‐(N‐succinimidyl succinylamino) ethyl]polyethylene glycol (NHSP) | Improve physical properties | Reagents may be toxic | Silk can be backbone and be functionalized to increase mechanical properties, covalent and noncovalent crosslinking possible through catechol oxidation and self assembly, respectively | [30] |
| *Crosslinking not applicable to bioink component alone, but included crosslinking methods for recent mixes that involve the component | ||||||
| Note: many bioink components can be methacrylated and made photosensitive outside of the common examples mentioned here (for example, Advanced BioMatrix includes collagen, gelatin, and hyaluronic acid) | [31] | |||||
Table 3.4: Commercially Available Bioinks (Not an Exhausting List)
| Bioink Component | SigmaAldrich | CellInk | Allevi | Brinter | Advanced Solutions | |||||
| Agarose | x | |||||||||
| Alginate | x | x | x | x | ||||||
| Chitosan | x | |||||||||
| Collagen | x | x | x | x | ||||||
| Decellularized ECM | x | |||||||||
| Fibrin/Fibrinogen | x | x | x | |||||||
| Gelatin | x | x | x | x | ||||||
| Gellan Gum | x | |||||||||
| Hyaluronic Acid | x | x | x | |||||||
| Hydroxyapatite | x | x | ||||||||
| PCL/PLA/PLGA | x | x | x | x | ||||||
| PEG | x | x | x | |||||||
| Pluronic F127 | x | x | x | x | ||||||
| Silk | x | x | ||||||||
| For crossreference to bioprinter table; Poietis, Fluicell, FELIX, 3DCultures, and CyfUses do not make specialty bioinks and therefore are not listed on this table. | ||||||||||
Table 3.5: Accessories
| Item | Uses | Acquisition |
| Syringe | Holds bioink, has removable plunger and can be filled up through tip (usually a Luer Lock tip) or from the top after removal of plunger | Varied depending on size that fits into specific bioprinter printhead |
| Cartridge | Holds bioink, has piston inside and must be filled up through tip (usually a Luer Lock tip) | |
| Metal Syringe | Holds bioink, made of stainless steel or aluminum for bioinks that require high printing temperatures | |
| Nozzle | Attaches to end of cartridge, tapered; results in less pressure and shear stress, reduced clogging, can be metal for high temperature applications | |
| Needle | Attaches to end of cartridge, blunted and straight; results in high pressure, minimal disturbance to ink, can be metal for high temperature applications | |
| Extrusion Printhead | Uses mechanical control to press bioink out; needs syringe with plunger | |
| Thermal Printhead | Heats and cools cartridge/syringe (most printheads include some temperature control, but speciality thermal printheads can include a larger temperature range) | |
| Electromagnetic Droplet Printhead | Uses electromagnetism to control droplet printing | |
| Pneumatic Printhead | Uses pressure to push bioink out; requires connection to compressed air source, needs cartridge with air adaptor on top and piston inside | |
| Thermoplastic Printhead | Uses controlled thermostat to print thermoplastics | |
| Photocuring Printhead | Uses UV light at any wavelength for controlled photocuring | |
| Female/Female Luer Lock Adapter | Connects cartridge to syringe, allows transfer/mixing of bioink | |
| Mixing Kit | Reduces bubble generation during cell and ink mixing |
[1] I. Noh, N. Kim, H.N. Tran, J. Lee, C. Lee, 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering, Biomater. Res. 23 (2019) 3. https://doi.org/10.1186/s40824-018-0152-8.
[2] S. Li, X. Tian, J. Fan, H. Tong, Q. Ao, X. Wang, Chitosans for tissue repair and organ three-dimensional (3D) bioprinting, Micromachines. 10 (2019). https://doi.org/10.3390/mi10110765.
[3] 3D Bioprinting: Bioink Selection Guide | Sigma-Aldrich, (n.d.). https://www.sigmaaldrich.com/technical-documents/articles/materials-science/3d-bioprinting-bioinks.html (accessed May 14, 2020).
[4] Y.R. Wang, J.M. Zheng, G.Y. Ren, P.H. Zhang, C. Xu, A flexible piezoelectric force sensor based on PVDF fabrics, Smart Mater. Struct. 20 (2011) 045009. https://doi.org/10.1088/0964-1726/20/4/045009.
[5] D. Wu, Y. Yu, J. Tan, L. Huang, B. Luo, L. Lu, C. Zhou, 3D bioprinting of gellan gum and poly (ethylene glycol) diacrylate based hydrogels to produce human-scale constructs with high-fidelity, Mater. Des. 160 (2018) 486–495. https://doi.org/10.1016/j.matdes.2018.09.040.
[6] Texas A&M University., Bioinks to print therapeutics in 3D: The new technology can be used for precise deposition of protein therapeutics — ScienceDaily, (n.d.). https://www.sciencedaily.com/releases/2019/06/190603124719.htm (accessed May 14, 2020).
[7] Z. Tan, C. Parisi, L. Di Silvio, D. Dini, A.E. Forte, Cryogenic 3D Printing of Super Soft Hydrogels, Sci. Rep. 7 (2017) 16293. https://doi.org/10.1038/s41598-017-16668-9.
[8] R. El Assal, S. Guven, U.A. Gurkan, I. Gozen, H. Shafiee, S. Dalbeyler, N. Abdalla, G. Thomas, W. Fuld, B.M.W. Illigens, J. Estanislau, J. Khoory, R. Kaufman, C. Zylberberg, N. Lindeman, Q. Wen, I. Ghiran, U. Demirci, Bio-inspired cryo-ink preserves red blood cell phenotype and function during nanoliter vitrification, Adv. Mater. 26 (2014) 5815–5822. https://doi.org/10.1002/adma.201400941.
[9] P.S. Gungor-Ozkerim, I. Inci, Y.S. Zhang, A. Khademhosseini, M.R. Dokmeci, Bioinks for 3D bioprinting: An overview, Biomater. Sci. 6 (2018) 915–946. https://doi.org/10.1039/c7bm00765e.
[10] R.G. Bai, K. Muthoosamy, S. Manickam, A. Hilal-Alnaqbi, Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering, Int. J. Nanomedicine. 14 (2019) 5753–5783. https://doi.org/10.2147/IJN.S192779.
[11] L.G. Gómez-Mascaraque, J.A. Méndez, M. Fernández-Gutiérrez, B. Vázquez, J. San Román, Oxidized dextrins as alternative crosslinking agents for polysaccharides: Application to hydrogels of agarose-chitosan, Acta Biomater. 10 (2014) 798–811. https://doi.org/10.1016/j.actbio.2013.10.003.
[12] K.Y. Lee, D.J. Mooney, Alginate: Properties and biomedical applications, Prog. Polym. Sci. 37 (2012) 106–126. https://doi.org/10.1016/j.progpolymsci.2011.06.003.
[13] C. Ryan, E. Alcock, F. Buttimer, M. Schmidt, D. Clarke, M. Pemble, M. Bardosova, Synthesis and characterisation of cross-linked chitosan composites functionalised with silver and gold nanoparticles for antimicrobial applications, Sci. Technol. Adv. Mater. 18 (2017) 528–540. https://doi.org/10.1080/14686996.2017.1344929.
[14] S. Abraham, D. Rajamanick, B. Srinivasan, Preparation, Characterization and Cross-linking of Chitosan by Microwave Assisted Synthesis, Sci. Int. 6 (2018) 18–30. https://doi.org/10.17311/sciintl.2018.18.30.
[15] Advanced BioMatrix – PhotoCol® (Collagen Only) #5198, (n.d.). https://advancedbiomatrix.com/photocol-only.html (accessed May 14, 2020).
[16] Z. Ahmad, J.H. Shepherd, D. V. Shepherd, S. Ghose, S.J. Kew, R.E. Cameron, S.M. Best, R.A. Brooks, J. Wardale, N. Rushton, Effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide concentrations on the mechanical and biological characteristics of cross-linked collagen fibres for tendon repair, Regen. Biomater. 2 (2015) 77. https://doi.org/10.1093/RB/RBV005.
[17] K.R. Siebenlist, D.A. Meh, M.W. Mosesson, Protransglutaminase (factor XIII) mediated crosslinking of fibrinogen and fibrin, Thromb. Haemost. 86 (2001) 1221–1228. https://doi.org/10.1055/s-0037-1616055.
[18] D. Hellio, M. Djabourov, Chemically and Physically Cross-linked Gelatin Gels, 2002. http://www.issp.u-tokyo.ac.jp/public/GelSympo2003/Proceedings/digitalproceedings/pdf_files/II_05Djabourov.pdf (accessed May 14, 2020).
[19] G. Yang, Z. Xiao, H. Long, K. Ma, J. Zhang, X. Ren, J. Zhang, Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods, Sci. Rep. 8 (2018) 1–13. https://doi.org/10.1038/s41598-018-20006-y.
[20] D.F. Coutinho, S. V. Sant, H. Shin, J.T. Oliveira, M.E. Gomes, N.M. Neves, A. Khademhosseini, R.L. Reis, Modified Gellan Gum hydrogels with tunable physical and mechanical properties, Biomaterials. 31 (2010) 7494–7502. https://doi.org/10.1016/j.biomaterials.2010.06.035.
[21] Z. Li, K.M. Bratlie, How Cross-Linking Mechanisms of Methacrylated Gellan Gum Hydrogels Alter Macrophage Phenotype, ACS Appl. Bio Mater. 2 (2019) 217–225. https://doi.org/10.1021/acsabm.8b00562.
[22] S. Khunmanee, Y. Jeong, H. Park, Crosslinking method of hyaluronic-based hydrogel for biomedical applications, J. Tissue Eng. 8 (2017). https://doi.org/10.1177/2041731417726464.
[23] M.N. Collins, C. Birkinshaw, Comparison of the effectiveness of four different crosslinking agents with hyaluronic acid hydrogel films for tissue-culture applications, J. Appl. Polym. Sci. 104 (2007) 3183–3191. https://doi.org/10.1002/app.25993.
[24] M. Mensitieri, L. Ambrosio, L. Nicolais, D. Bellini, M. O’Regan, Viscoelastic properties modulation of a novel autocrosslinked hyaluronic acid polymer, J. Mater. Sci. Mater. Med. 7 (1996) 695–698. https://doi.org/10.1007/BF00123409.
[25] N. Yui, T. Okano, Y. Sakurai, Photo-responsive degradation of heterogeneous hydrogels comprising crosslinked hyaluronic acid and lipid microspheres for temporal drug delivery, J. Control. Release. 26 (1993) 141–145. https://doi.org/10.1016/0168-3659(93)90113-J.
[26] M. Azami, M. Rabiee, F. Moztarzadeh, Glutaraldehyde crosslinked gelatin/hydroxyapatite nanocomposite scaffold, engineered via compound techniques, Polym. Compos. 31 (2010) 2112–2120. https://doi.org/10.1002/pc.21008.
[27] S. Wang, M.J. Yaszemski, J.A. Gruetzmacher, L. Lu, Photo-crosslinked poly(ε-caprolactone fumarate) networks: Roles of crystallinity and crosslinking density in determining mechanical properties, Polymer (Guildf). 49 (2008) 5692–5699. https://doi.org/10.1016/j.polymer.2008.10.021.
[28] S. Lee, X. Tong, F. Yang, Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release, Biomater. Sci. 4 (2016) 405–411. https://doi.org/10.1039/c5bm00256g.
[29] Pluronic – an overview | ScienceDirect Topics, (n.d.). https://www.sciencedirect.com/topics/chemistry/pluronic (accessed May 14, 2020).
[30] N. Suttenun, P. Punyamoonwongsa, A New Crosslinker for the Preparation of Silk Fibroin Hydrogels, Macromol. Symp. 354 (2015) 273–279. https://doi.org/10.1002/masy.201400097.
[31] D.L. Heichel, K.A. Burke, Dual-Mode Cross-Linking Enhances Adhesion of Silk Fibroin Hydrogels to Intestinal Tissue, ACS Biomater. Sci. Eng. 5 (2019) 3246–3259. https://doi.org/10.1021/acsbiomaterials.9b00786.
4. Different Ways to Bioprint
Approaches
Inkjet-based (droplet-based)
-
- Deposits droplets of bioink on hydrogel or culture dish
- Thermal, piezoelectric, or electrostatic actuator
- Advantages: high resolution, high printing speed, low cost, possibility of cell concentration gradients
- Disadvantages: can only use low viscosity inks, nozzle clogging, poor vertical structure
- Biomaterials ex. Cell suspension, alginate, collagen, fibrin, thrombin, fibrinogen
- Cell ex. Porcine Schwann cells, neuronal analogs, cardiomyocytes, microvascular endothelial cells, fibroblasts, keratinocytes
Extrusion
-
- Pneumatic, piston, or screw-based pressure extrude bioink to for layer-by-layer constructs
- Wide range of materials possible
- Includes fused deposition bioprinting, where a solid filament is passed through a heating element to then be printed
- Advantages: scalability, high viscosity bioinks, high cell concentration
- Disadvantages: lower resolution, shearing force can damage cells, nozzle clogging
- Biomaterials ex. Salicylic acid, collagen, alginate, PLA fibers, GelMA, hyaluronic acid, fibrinogen, glycerol, poly(urethane), poly(caprolactone), decellularized adipose tissue, PEG, pluronic
- Cell ex. Chondrocytes, HUVECs, fibroblasts, keratinocytes, adipose-derived SC, respiratory endothelial cells, osteoblasts
Laser-assisted
-
- Uses a laser pulse and donor layer made of a ribbon coated with liquid biological materials to deposit droplets on the receiving substrate
- The laser creates an expanding vapor bubble due to vaporization of the energy absorbing layer until the bubble bursts to produce printed drops of bioink
- Advantages: high cell viability (due to non-contact process), no clogging, high resolution, can print in high cell density, can print low viscosity
- Disadvantages: cellular damage from laser, limited scalability
- Biomaterials ex. Human osseous cell sheets, collagen
- Cell ex. HUVECs, fibroblasts, keratinocytes, keratinocytes, hMSCs
- Other printed material ex. DNA, peptides
Stereolithography
-
- Uses accurate light projection to crosslink photosensitive polymers in a layer-by-layer fashion
- Can use UV or visible light
- Advantages: high resolution, no clogging, can use high cell concentrations
- Disadvantages: can only use photo-responsive bioinks, UV damage to cells
- Biomaterials ex. PEGDA, GelMA, graphene nanoplatelets, nHA
- Cell ex. Breast cancer cells, HUVECs, skeletal muscle cells, osteoblasts, fibroblasts, mesenchymal cells
Extended Reading
- Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. 2016;14:271. Published 2016 Sep 20. doi:10.1186/s12967-016-1028-0
- Kačarević ŽP, Rider PM, Alkildani S, et al. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials (Basel). 2018;11(11):2199. Published 2018 Nov 6. doi:10.3390/ma11112199
5. Challenges in Bioprinting
- Vascularization
- Lymphatics
- Nerve system
- Complex Tissue Structure (e.g. to achieve diversity of cell types and mechanical diversity). Examples include:
- Mechanical diversity in cartilage
- Muscle-tendon interface
- Layering in skin, cornea, retina, and trachea
- Challenges for Translational Research
- Scaffolding degrading at a proper rate
- Scalability
- Cell sources
Extended Reading
- V. Murphy, P. De Coppi, A. Atala, Opportunities and challenges of translational 3D bioprinting, Nat. Biomed. Eng. 4 (2020) 370–380. https://doi.org/10.1038/s41551-019-0471-7.
- Masaeli, C. Marquette, Direct-Write Bioprinting Approach to Construct Multilayer Cellular Tissues, Front. Bioeng. Biotechnol. 7 (2020). https://doi.org/10.3389/fbioe.2019.00478.
6. Current Approaches to Creating Vessels and Challenges
Challenges
- Complex branching pattern with varying diameter
- Small vessels require high printer resolution
Strategies
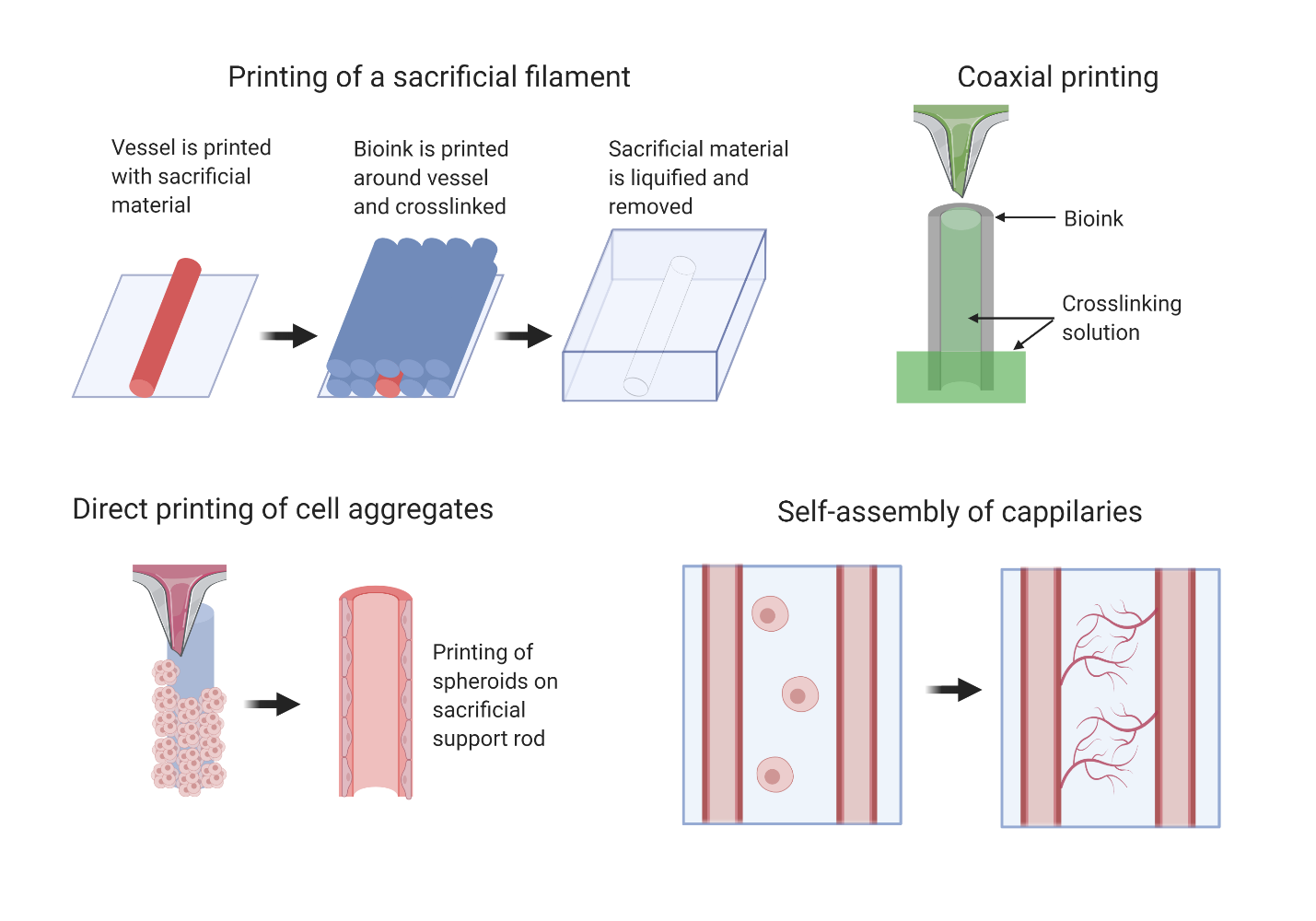
Printing a sacrificial filament within a gel that is removed after crosslinking of the bulk structure
Ex. Printing a Pluronic-F127 within a Pluronic F127-diacrylate construct, performing photocrosslinking, then lowering temperature to liquify the Pluronic-F127
Coaxial extrusion that prints onto a stage that lowers into a crosslinking bath
Ex. A nozzle has interior flow of calcium solution and exterior flow of alginate. Interior flow of calcium crosslinks the interior of the vessel wall and the crosslinking bath crosslinks the exterior of the vessel.
Direct printing of cell aggregates
Ex. Printing cell spheroids on sacrificial agarose support rods
Patterning of cells into structures for self-assembly by use of bioactive inks
Ex. Printing endothelial cells between channels to create capillary network between the channels, use of peptides and growth factors
Fabrication of free-standing tubular structures
Ex. Ink-jet printing of an alginate bioink into a CaCl2 solution
Extended Reading
- Richards, D., Jia, J., Yost, M., Markwald, R., & Mei, Y. (2017). 3D Bioprinting for Vascularized Tissue Fabrication. Annals of biomedical engineering, 45(1), 132–147. https://doi-org.proxy1.library.jhu.edu/10.1007/s10439-016-1653-z