1. Engineer Quantum-Enabled Dial (QED) proteins to modulate biochemical reactions in cells.
Though the role of magnetic fields in biology is relatively understudied, recent evidence suggests that magnetic fields may modulate important functions in cells. It has been observed that magnetic fields modulate the spin state of radical pairs generated via chemical or photonic excitation in many protein species. One of the prominent examples lies in flavoproteins. Magnetic fields can affect the ratio between triplet and singlet radical pair subpopulations, leading to different yields of downstream reaction products. This has important physiological implications, including circadian rhythms and DNA repair. Inspired by the spin chemistry of magnetosensitive flavoproteins, we developed a class of synthetic protein, quantum-enabled dials (QEDs), to actuate enzymatic activities by manipulating the spin state via the combination of light and magnetic fields. Through QEDs, enzymatic activities can be fine-tuned in a graded manner by adjusting the strength of externally applied magnetic fields. QEDs can broaden the application space of magnetism in medicine.
2. Study the effects of long-range and high forces generated by cancer cells on tumor microenvironment.
Some types of cancer cells can generate higher forces compared to their normal counterparts. The high forces can be transmitted over distance at the order of hundreds of microns. There can be two possible effects: (a) activation of mechanosignaling pathways in cells that are not in the immediate proximity of the cancer cells; and (b) mechanical remodeling of the microenvironment as a result of reorganization of fibrillar ECM proteins (see movie below) and subsequent changes in the diffusion rate of secreted substances such as cytokines, microRNAs and exosomes.
Figure: The spheroid consisting of early-stage-cancer cells (center)exerts stronger forces than the control spheroids made of normal cells (left) or cancer cells expressing RhoA mutations. Jung et al. (2020) Biomaterials
We are looking into how these effects contribute to cancer pathology.
3. Construct physiologically representative organoid systems by mimicking in vivo biophysical characteristics.
In order to have a better representation of physiological conditions, we are interested in developing an organoid system that takes into account the in vivo biophysical factors such as ECM organization, rigidity, viscosity of the interstitial fluid, and so on. Hopefully the pharmaceutical test results derived from such organoid system can better predict the outcomes of clinical trials than the currently prevailing cell culture model systems do.
Figure: Metastasis-on-a-chip platform which consists of organoids and bioprinted vasculature is used to study metastasis.
4. Build instruments to study classical and quantum mechanics in biological systems
We design and build instruments allowing us to studying the responses of cells and/or tissues to mechanical stimuli or lack thereof. One example is the universally fitted, real-time imaging capable stretcher. We can directly monitor fast cell responses at high resolution using the stretcher.
Figure: The design of a cell stretcher for high-magnification microscopy.
We also build microgravity simulators where with a one- or two-axis rotation, cultured tissues are accelerated until reaching terminal velocity at which the gravitational force is counterbalanced by the hydrodynamic forces. The simulator has the capacity to measure cardiac tissue contractility and apply pulsative tensile forces to simulate heart beats. It will help us understand how the heart is impacted by the microgravity in the space and whether we can rehabilitate the cardiac functions by externally applying forces longitudinally.
We also built Halbach array to generate patterned magnetic fields to control activities of magnetosensitive proteins in tissues.
We welcome inquiries from potential collaborators for possible applications of our instruments. Please email Yun Chen (yun.chen@jhu.edu) for details.
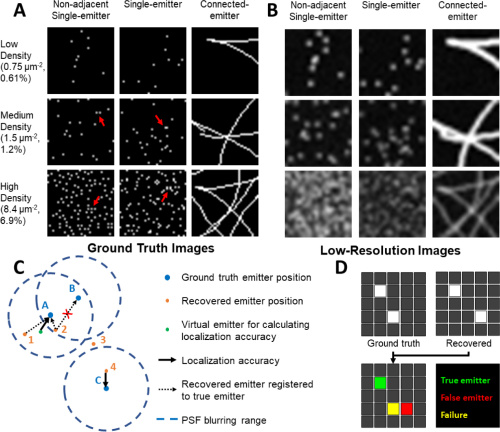
5. Develop imaging platforms and apply computational super-resolution to enhance image qualities.
Recently developed tools in biochemistry, molecular biology, biophysics contribute greatly to advancing our understanding how molecules interact with each other in healthy and abnormal cells. However, sometimes what we know at the molecular level cannot be linearly scaled up to the tissue level; and such “not seeing the wood for the trees” issue arises from the fact that heterogeneity of the tissue is not accounted for. In order to understand the inter-scale relationship between molecules, cells and tissues in homeostasis and pathology, we will address the issue by developing a new imaging platform and corresponding probes to survey the tissue heterogeneity at single-molecule level by combining coherent diffraction and compressed sensing for image reconstruction achieving sub-wavelength resolution.
Figure: Ground truth images and corresponding low-resolution images for testing reconstructing algorithms, with schematics for performance evaluation metrics. Chen and Chen. (2021) Biomedical Optics Express.
6. Explore the effects of high extracellular fluid viscosity on cells.
Cells are surrounded by and move through biological fluids that span orders of magnitudes in viscosity in vivo, including mucus, saliva, and synovial fluid, among others. Interstitial fluid in tumor microenvironment is viscous, and the mucus of patients with airway disorders is highly viscous. Even in healthy individuals, fluctuations in lipid and protein secretion and hydration levels can lead to changes in local fluid viscosity. However, our understanding of whether cells actively respond to viscosity changes and which signaling pathways are involved is very limited. Compared to other biophysical parameters, such as substrate stiffness, hydrostatic pressure, or topography, for example, the effects of viscosity on cell behavior remain unexplored. Recently, we observed that some adherent cells migrated at higher speeds in highly viscous medium. This is a counterintuitive observation with profound biological and health implications. For example, elevated viscosity in the tumor microenvironment and in ascites can increase the rate of cancer cell motility and promote metastasis. However, we are only scratching the surface of the profound biophysical effects of extracellular fluid viscosity. We are investigating how gene expression, proliferation, cell-cell interactions are affected.
Video: A cancer cell changes its shape and becomes flat immediately after the viscosity is increased to 1000 times higher. Pittman et. al. (2022) Nature Physics.
See more details at https://scholar.google.com/citations?hl=en&user=0jZf7TMAAAAJ&view_op=list_works&sortby=pubdate